Clinical trials Day 2022: How data can make trials faster, more efficient and better at improving care
20 May 2022
We explore how routinely collected health data may hold the key to super-charging how clinical trials are delivered, and in turn, how quickly we can bring improvements to people's lives.

Clinical trials are a vital tool for advancing medical knowledge and, in turn, patient care. By randomly assigning people to receive treatments or medical interventions and comparing the results, they represent the gold standard for determining the best ways to improve health and care.
The pandemic has meant public understanding of the importance of clinical trials is at an all-time high – with landmark trials delivering treatments, vaccines and care for those critically ill with COVID-19 in record time.
The success of trials like these depends on huge amounts of resource, money and time. In order to be sure a treatment or intervention is effective and safe, data from many hundreds – and often thousands – of people are needed. And collecting trial-specific data throughout the trial can also be incredibly resource intensive, requiring many hours of dedicated research staff time.
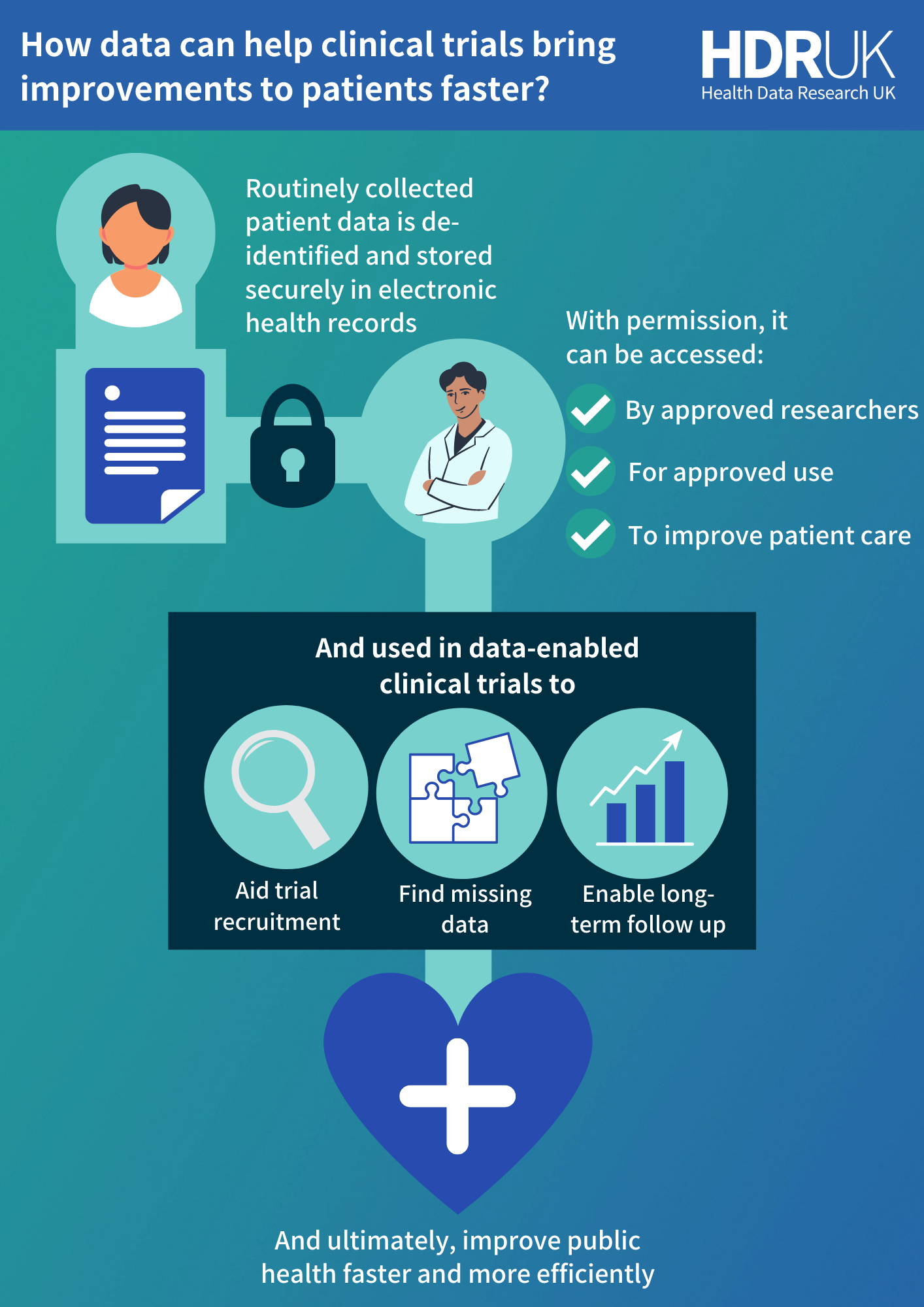
Overcoming these challenges to deliver improvements to healthcare faster and more efficiently requires a universally streamlined approach to clinical trial processes. At HDR UK, we believe the key to this may lie in routinely collected electronic health records.
An opportunity for better, faster and more efficient clinical trials
Electronic health records are the digital version of your medical record, collected and added to as you interact with the healthcare system – from GP notes, hospital visits, prescriptions and more.
Much of this information overlaps with the data that is needed for clinical trials. It makes electronic health records a valuable resource with the potential to improve efficiencies and reduce the data collection burden of these studies, and ultimately helping to bring better care to patients faster.
For example, by allowing approved researchers to securely and lawfully access information about a group of patients with a certain medical condition, they can quickly identify those who might be eligible for a clinical trial to find new ways to treat that condition. And by already having additional information about their age, medical history, lifestyle and more, the researchers can get the data they need to understand the safety and efficacy of the treatment without repeating time-consuming data collection.
Electronic health records are also a live data resource, constantly being updated throughout a person’s life. With the permission of the patients, this means they can continue to be used even after a trial finishes to monitor patients long-term.
Putting it into practice: using electronic health records to answer long-forgotten research questions
In a randomised clinical trial carried out in English hospitals between August 1993 and October 2001, researchers set out to understand whether infants that had been fed nutritionally enriched formula saw any cognitive benefits compared to infants who were fed standard formulas.
The team gathered data from 1,763 children over several years, who were given either enriched or standard formula milk. However, due to the long-term follow up needed to prove whether the enriched formula-fed children saw any cognitive improvements, the trial was deemed inconclusive.
The research lay dormant for over a decade, but in 2018 researchers from UCL Great Ormond Street Institute of Child Health and the UCL Institute of Education saw an opportunity to reopen it by tapping into electronic health records held by the National Pupil Database (NPD).
The NPD provides approved researchers with access to anonymised pupil data for the purpose of improving the education standards and quality in UK schools. Supported by HDR UK, the team found they could link 91.2% of the historic trial data with data from the NPD about the children’s long-term education outcomes. It made it possible to reopen the trial – marking it the first reactivated study of its kind in UK children.

In an interview with UCL, lead author Dr Maximiliane Verfürden added: “No previous research has shown conclusive benefits for cognitive ability of these nutritionally modified formulas, but the evidence was uncertain due to short follow-up periods and high drop-out rates over time
“By linking data from historic randomised trials to data routinely collected from school tests, the study provides the best available evidence on whether these formula modifications affect children’s cognitive ability.
“This study sets a precedent for other trials and cohorts to use linkage of historic trials to administrative data to address high participant drop-out over time and answer important questions about long-term outcomes in children and young people.”
The results found the enriched infant formula did not promote long-term cognitive benefit when compared with standard infant formulas, allowing researchers to finally answer the long-standing research question.
What’s next for data enabled clinical trials?
Using electronic health records to re-activate dormant trials is just one of many potential uses of this kind of data to gain insights from clinical trials faster and more efficiently. At the start of the pandemic, routinely collected patient data was used to help set up the ground-breaking RECOVERY trial in Oxford in just nine days, and helped the team identify the world’s first treatment for COVID-19.
But there are still barriers to making all clinical trials ‘data enabled’. Dr Marion Mafham, who led the data linkage in the RECOVERY trial, explained: “Data linkages [between electronic health records and standard clinical trial data] aren’t straight forward to set up. We were lucky at Oxford to have lots of in-house expertise, but most trial teams don’t have access to the knowledge of how to collect, use and interpret this kind of data.
“Another challenge is convincing people this approach is robust. Clinical trials have been done in the same way for decades – using carefully collected research-specific data with a lot of source data verification. It’s very laborious, but it’s tried and tested and has formed the basis of thousands of treatment approvals to date.
“Moving away from this system would be a major shift.”
HDR UK’s Better, Faster and More Efficient Clinical Trials programme is aiming to support this step-change. The programme provides training and resources to help researchers learn how to utilise routine clinical data, but it’s also looking to support studies that compare newer data methods with traditional ones. Work like this could help provide evidence to regulators, industry and funders that these methods are reliable.