Data experts call for better use of routine health data in clinical trials
20 July 2022
In a commentary published today, HDR UK researchers describe how a key roadblock to using the UK’s rich resources of health data can be removed to make clinical trials faster and more efficient.
Clinical trials can take many years and are expensive to run, due in-part to the huge amounts of data necessary to prove the effectiveness of new treatments and healthcare interventions.
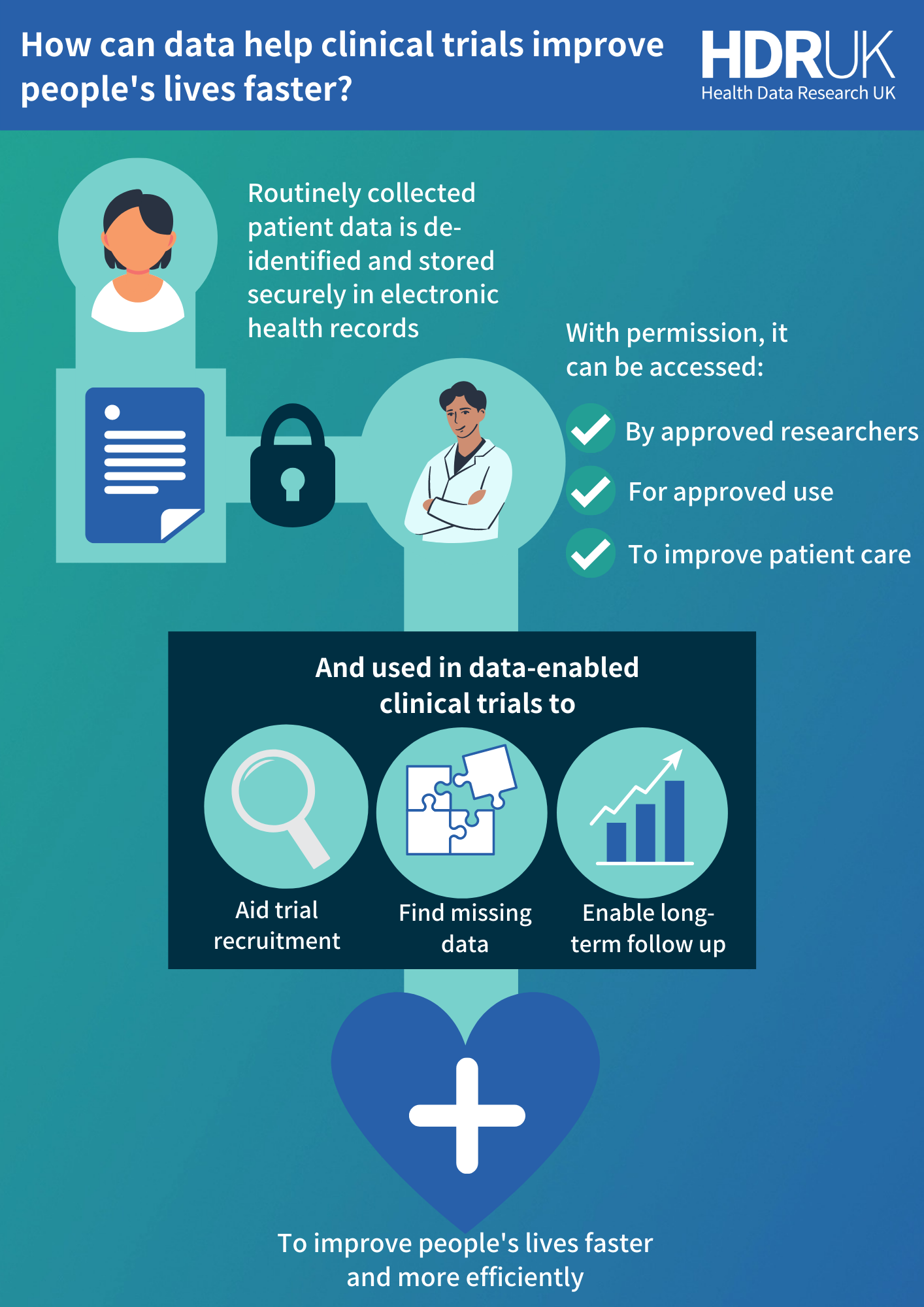
In the commentary, and an additional detailed report, the researchers explain steps that can be taken to make use of data that is already collected as patients interact with the healthcare system – like GP notes, prescriptions and hospital data – in clinical trials.
Looking at the data given to trials from registries between 2013 and 2018, fewer than 5% of UK-based randomised clinical trials made use of routinely collected health data.
To combat this, the Healthcare Systems Data for Clinical Trials Collaborative Group have developed a process to document the provenance (information on the origin of data) and integrity (information on the quality of data) of routinely collected healthcare datasets. These are two key elements that healthcare regulators look at to determine if the results of clinical trials can go on to influence how patients are treated and cared for.
The team applied the methods to two NHS Digital datasets, demonstrating that both are sufficiently reliable to be used in clinical trials.
In a joint statement released today, the researchers say: “Healthcare systems data has the potential to transform the conduct of clinical trials, and many trialists intend to use them more for recruitment, outcomes, and follow-up. There are excellent examples of trials that have benefitted from their use, such as the RECOVERY and PRINCIPLE trials investigating potential treatments for COVID-19.
“But a key challenge is that trial sponsors need to confirm the provenance and integrity of these data to satisfy regulatory standards. The collaborative group led by our Conduct Methodology team, demonstrated the integrity of two important NHS Digital datasets in our report, and this has now been highlighted through our commentary in August’s issue of Lancet Digital Health.
“We urge data providers to take these necessary steps to support assessments of provenance and integrity for all relevant datasets. This step will make trials more efficient and consequently lead to faster improvements in healthcare for all.”
How does routinely collected healthcare data improve clinical trials?
The Healthcare Systems Data for Clinical Trials Collaborative Group
Medical Research Council Clinical Trials Unit, University College London: Macey Murray, Sharon Love, James Carpenter, Max Parmar, Matt Sydes.
NHS Digital: Suzanne Hartley, Heather Pinches.
Big Data Institute, University of Oxford: Martin Landray, Marion Mafham.